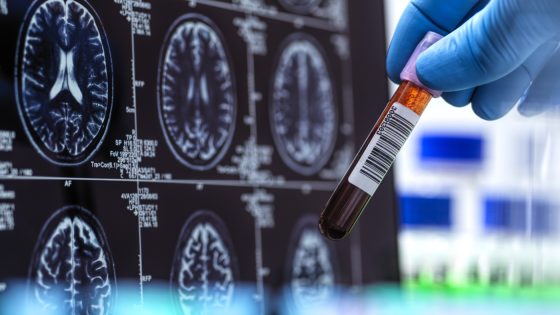
A groundbreaking Alzheimer’s blood test has been cleared by the Food and Drug Administration, making diagnosis significantly more accessible and accurate. This advancement is set to transform how doctors approach Alzheimer’s treatment, especially for patients aged 55 and older who exhibit cognitive symptoms.
- First FDA-cleared Alzheimer's blood test available
- Targets individuals aged 55 and older
- Detects amyloid plaques in the brain
- Improves diagnostic accuracy for primary care
- Expected to increase early Alzheimer's diagnoses
- Guidelines for patient counseling to be released
The test detects amyloid plaques in the brain, which are indicative of Alzheimer’s disease. With this new tool, primary care physicians can achieve a diagnosis accuracy rate of over 90%, compared to the current 60% accuracy in general practice. As a result, many more individuals may receive timely diagnoses, allowing for earlier intervention and treatment options.
This innovative blood test raises an important question: how will it impact the lives of those at risk for Alzheimer’s? With the potential to democratize testing, it offers hope for earlier diagnosis and intervention. Consider these health recommendations:
- Discuss cognitive symptoms with your healthcare provider.
- Stay informed about new diagnostic tools and treatments.
- Participate in regular health screenings, especially if over 55.
As we look ahead, embracing these advancements in Alzheimer’s diagnostics can empower patients and families to make informed health decisions and improve quality of life.