Health Secretary Robert F. Kennedy Jr. recently stirred discussions by advocating for expanded access to experimental therapies, despite acknowledging their potential risks. On a podcast with longevity expert Gary Brecka, he expressed his intention to challenge the FDA’s stance on alternative medicine.
- Robert F. Kennedy Jr. supports experimental therapies.
- He acknowledges risks of fraudulent treatments.
- Plans to end FDA's opposition to alternative medicine.
- Advocates for patient choice in experimental drugs.
- Cites personal success with stem cell treatment.
- Experts warn against lack of regulatory safeguards.
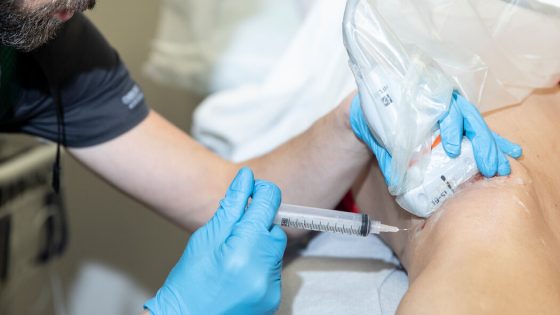
Kennedy emphasized that individuals should have the freedom to try experimental drugs. He cited his own positive experience with stem cell treatment for his neurological condition, underscoring the need for more options in healthcare. His remarks, made on June 5, 2025, raise questions about the balance between patient autonomy and safety.
As the conversation around alternative medicine grows, a key concern arises: how can we ensure patient safety while promoting innovation? While Kennedy’s perspective highlights the importance of choice, it also opens the door to potential exploitation. Consider these recommendations:
- Consult healthcare professionals before pursuing experimental treatments.
- Research the credibility of clinics offering alternative therapies.
- Stay informed about FDA regulations and safety warnings.
- Evaluate the risks versus benefits of any treatment option.
As we navigate this evolving landscape, it’s crucial to advocate for informed choices and prioritize safety in healthcare decisions.